We are striving to develop new breakthrough drugs to help patients combat diseases for which currently there are no appropriate therapeutic drugs. To this end, we conduct a venture-like drug research approach, which is licensing-out compounds at early stages discovered through in-house exploratory research to highly specialized domestic and overseas companies. To expand our pipeline and incorporate AI and other rapidly evolving digital technologies in our drug discovery methods, we have been pursuing alliances with companies and research institutions that are conducting cutting-edge research in their respective fields.
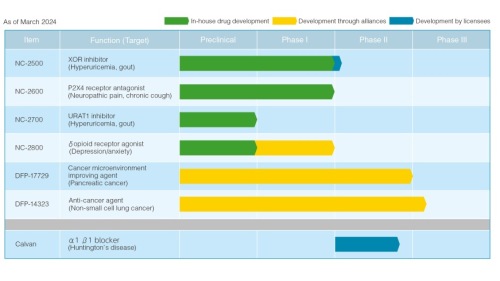
NC-2800, a δ Opioid Receptor Agonist for depression and anxiety
NC-2800 is a chemical compound with strong potential as an anti-depression and anti-anxiety treatment that the Company discovered through collaborative study with the University of Tsukuba, Kitasato University, and the National Center of Neurology and Psychiatry. The Japan Agency for Medical Research and Development (AMED) selected this compound for inclusion under its industry–academia collaboration program in 2015 and, with the agency’s support, we conducted preclinical trials. As a result, the compound received high acclaim for its potential as a therapeutic drug candidate.
In 2018, it was selected as a AMED’s CiCLE project* for public funding and support, which helped facilitate the launch of a phase Ⅰ trial in July 2021. Phase I trial was completed in FY2023, and since there were no major safety issues and tolerability was ensured, we are currently preparing to conduct phase IIa.
In June 2021, we concluded a collaborative research and development agreement and an option agreement for NC-2800 with Sumitomo Pharma Co., Ltd. Thus, Sumitomo Pharma is participating in the CiCLE project as a collaborating institution.
While each year sees more patients with mood disorders, such as depression and anxiety, only about 40% of the patients are satisfied with the treatment they receive using drugs. NC-2800 is expected to be a first-in-class drug with an excellent balance between safety and efficacy, and minimal impact in terms of side effects.
* Development of innovative mood regulators with a mechanism of action based on activating opioid δ receptors; AMED is providing support from March 30, 2018, until March 31, 2028
NC-2600, a P2X4 Receptor Antagonist for neuropathic pain and chronic cough
In joint research with Kyushu University, we have carried out studies for a drug candidate with a novel mechanism of action to treat neuropathic pain. Since FY2012, the Company has been advancing research and development with the support of the Japan Science and Technology Agency, taken over by AMED in FY2015. With the support of AMED, we completed phaseⅠ trials in FY2017.
In FY2020, we added chronic cough to the primary indications of the compound. While moving to enhance its value by collaborative research for various indications, we have been conducting out-licensing activities of the compound to domestic and overseas companies.
NC-2500, an XOR Inhibitor for gout and hyperuricemia
Current drug therapies for lowering uric acid pose a risk of causing an acute attack of gout, due to the sharp decrease in uric acid levels. However, results from NC-2500 phase I trial showed its unique property to gradually lower blood uric acid levels, suggesting possibilities to rectify this issue. In February 2023, we signed a licensing agreement with a Chinese company, which is working to develop the drug in China.
In addition, data indicates that NC-2500 is effective against neurodegenerative disorders such as Alzheimer’s disease. So we are exploring the possibility of expanding the indication of NC-2500 to include such disorders.
NC-2700, a URAT 1 Inhibitor for gout and hyperuricemia
To promote the excretion of uric acid from the body, NC-2700 inhibits the transporter URAT-1, which re-absorbs the uric acid by the kidneys. The mechanism of action is different from the above NC-2500.
Non-clinical studies have shown that NC-2700 facilitates the excretion of uric acid and also ameliorates aciduria, thereby helping to prevent kidney damage and kidney stones, which are concerns when uric acid is excreted. We seek to out-license the compound to domestic and overseas companies.
DFP-17729, a Cancer Microenvironment Improving Agent for pancreatic cancer
In March 2020, we concluded a licensing agreement for DFP-17729, with Delta-Fly Pharma. DFP-17729 is expected to generate groundbreaking therapeutic effects for refractory cancer by alkalizing the acidic environment around tumors, that facilitates cancer growth.
The compound is currently developed by Delta-Fly Pharma for end-stage pancreatic cancer and completed phase Ⅱ trials in FY2022, which compared survival time between combination with chemotherapy and chemotherapy alone. Data analysis for efficacy and safety is underway to prepare for the next phase of trials.
DFP-14323, an antineoplastic drug for non-small cell lung cancer
Lung cancer is the fourth-most common cancer by site both in men and women. A National Cancer Center Research Institute survey* shows that, in 2019, some 120,000 cases were diagnosed as lung cancer in Japan. In 2022, lung cancer caused 76,000 deaths in Japan, which is the highest number by site.
In March 2022, we concluded a licensing agreement with Delta-Fly Pharma for DFP-14323. The compound targets epidermal growth factor receptor (EGFR) mutation positive non-small cell lung cancer.
Previous studies have suggested that DFP-14323 strengthens the immune response of cancer patients by binding to aminopeptidase N, which is found on the surface of cancer immunocompetent cells. In this way, the compound reduces the dosage required of standard anticancer drugs and enhances their efficacy without increasing the side effects. That makes DFP-14323 a promising therapeutic agent, especially for late-stage and elderly cancer patients.
PhaseⅡ trials for EGFR mutation positive non-small cell lung cancer at stages 3 and 4 have been completed. The results were presented at the American Society of Clinical Oncology meeting held in June 2022. Again the results demonstrated the agent’s efficacy.
In February 2024, phase Ⅲ trial has been started.
* National Cancer Center Research Institute, Cancer Statistics in Japan 2023.
| Business Development We are interested in any opportunities of collaboration, partnering or licensing of our pipeline products and welcome any proposal on them. If you have any interest in any of our pipeline products, please contact our business development team: bd@chemiphar.co.jp. |